Nonalcoholic Steatohepatitis Clinical Trial and Pipeline Insight Report 2023 | 100+ Key Companies Investigating Their Lead Candidates to Improve the Treatment Space
Nonalcoholic steatohepatitis (NASH) has the potential to be a large and lucrative therapy market owing to the prevalence of the disease and the lack of approved agents. Patients with NASH are at risk of liver fibrosis, cardiovascular disease, and cancer. The growing prevalence of diseases in the region is creating favorable growth opportunities for drugmakers. Key regional manufacturers in the industry are investing in R&D activities to boost the development of novel drugs for nonalcoholic steatohepatitis (NASH) treatment. There are no approved therapies for NASH, which creates a significant unmet need due to the high clinical and economic burden on healthcare systems and the large prevalent population. There is a pressing need for new, effective drug treatments for NASH.
DelveInsight’s ‘Nonalcoholic Steatohepatitis Pipeline Insight 2023‘ report provides comprehensive global coverage of available pipeline nonalcoholic steatohepatitis therapies in various stages of clinical development, major pharmaceutical companies are working to advance the NASH pipeline space and future growth potential of the nonalcoholic steatohepatitis pipeline domain.
Nonalcoholic Steatohepatitis Pipeline Report Key Takeaways
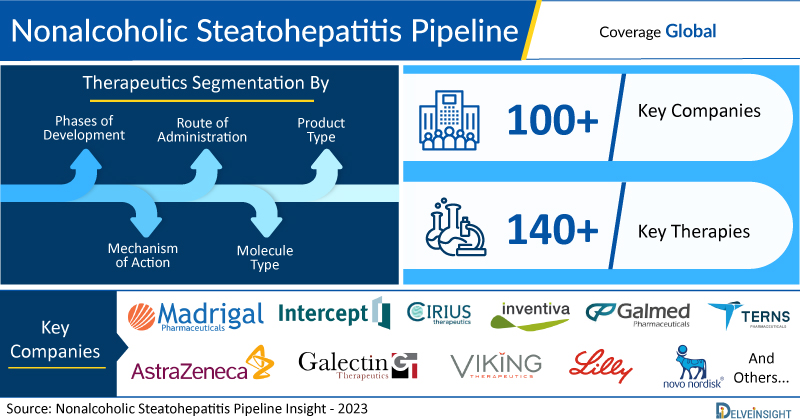
- DelveInsight’s nonalcoholic steatohepatitis pipeline report depicts a robust space with 100+ active players working to develop 140+ pipeline therapies for nonalcoholic steatohepatitis treatment.
- Key nonalcoholic steatohepatitis companies such as Madrigal Pharmaceuticals, Intercept Pharmaceuticals, Cirius Therapeutics, Novo Nordisk, Inventiva, Galmed Pharmaceuticals, AstraZeneca, Galectin Therapeutics, Viking Therapeutics, Eli Lilly and Company, Terns Pharmaceuticals, Sinew Pharma, 89bio, Inc., Eccogene, Novartis Pharmaceuticals, Poxel SA, AngioLab, Pfizer, Arrowhead Pharma, LG Chem, Redx Pharma, Lipocine, Inc., CytoDyn, Inc., Alnylam Pharmaceuticals, Inc., Chemomab Therapeutics, NuSirt Biopharma, HK inno. N, Aligos Therapeutics, Altimmune, Inc., Kowa Pharmaceutical, Ionis Pharmaceuticals, NorthSea Therapeutics, Rivus Pharmaceuticals, Hanmi Pharmaceutical, Hepagene Therapeutics, HighTide Biopharma, Akero Therapeutics, Merck Sharp & Dohme LLC, Cascade Pharmaceuticals, Hepion Pharmaceuticals, Chipscreen Biosciences, Boston Pharmaceuticals, Bristol-Myers Squibb, Sunshine Lake Pharma, GSK plc., Future Medicine, Gilead Sciences, ENYO Pharma, Histogen, and others are evaluating new nonalcoholic steatohepatitis drugs to improve the treatment landscape.
- Promising nonalcoholic steatohepatitis pipeline therapies such as TERN-101, SNP-610, LJN452 (tropifexor + licogliflozin), PXL065, ALS-L1023, PF-06865571 + PF-05221304, PF-06835919, PF-06865571, ORMD-0801, Norucholic acid, NNC0194-0499, MN-001, MK-3655, MET642, MET409, LPCN 1144, LIK066, Leronlimab, ALN-HSD, RXC007, LR19018, LR19131, CM101, Leu-Mit-Sil (NS-0200), IN-A010, Efruxifermin (EFX), Efinopegdutide, JKB-122, CS0159 (Linafexor), CRV431, Chiglitazar sodium, ARO-PNPLA3, ECC4703, BOS-580, HEC96719, GSK4532990, BIO89-100, Cotadutide solution, Firsocostat, EPY001a, Emricasan, BMS-986036, BIO89-100, BI 456906, BFKB8488A, AXA1125, ASC 41, GS-9674 (Cilofexor), ZSP1601, and others are under different phases of nonalcoholic steatohepatitis clinical trials.
- In April 2023, Madrigal Pharmaceuticals announced that resmetirom has received Breakthrough Therapy designation from the U.S. Food and Drug Administration (FDA) for the treatment of patients with NASH with liver fibrosis. The Company also announced that the outcomes portion of the Phase 3 MAESTRO-NASH biopsy trial has completed enrollment.
- In March 2023, PathAI announced its partnership with GSK (LSE/NYSE: GSK) on HORIZON, a randomized Phase 2b non-alcoholic steatohepatitis (NASH) clinical trial (NCT05583344). The trial will measure improvements in liver histology with GSK4532990 compared with placebo in participants with NASH and advanced fibrosis. PathAI’s role will be to generate, digitize, and analyze liver biopsy slides for central pathologist evaluation in addition to AI-powered histologic evaluation using PathAI’s AI-based Measurement of NASH Histology (AIM-NASH) tool1. AIM-NASH metrics will be included as exploratory endpoints in this study.
- In March 2023, Aligos Therapeutics presented Phase 1 data for its thyroid receptor-beta (THR-ß) agonist candidate, ALG-055009, at the 15th Paris Hepatology Conference, taking place virtually March 27 – 29, 2023.
- In March 2023, POXEL SA announced publication in Journal of Hepatology of positive results for DESTINY-1 (Deuterium-stabilized R-pioglitazone [PXL065] Efficacy and Safety Trial In NASH), a 36-week dose-ranging Phase 2 trial. PXL065 is a novel, proprietary deuterium-stabilized R-stereoisomer of pioglitazone which has reduced PPARγ activity, but retains non-genomic pioglitazone actions.
- In January 2023, Intercept Pharmaceuticals announced that the U.S. Food and Drug Administration (FDA) has accepted Intercept’s New Drug Application (NDA) for obeticholic acid (OCA) seeking accelerated approval for the treatment of patients with pre-cirrhotic liver fibrosis due to nonalcoholic steatohepatitis (NASH).
Request a sample and discover the NASH drugs in development 2023 @ Nonalcoholic Steatohepatitis Pipeline 2023 Report
The nonalcoholic steatohepatitis pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage nonalcoholic steatohepatitis drugs, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the nonalcoholic steatohepatitis clinical trial landscape.
Nonalcoholic Steatohepatitis Overview
Nonalcoholic steatohepatitis (NASH) is a type of liver inflammation and damage caused by fat accumulation. It is one of the numerous disorders known as nonalcoholic fatty liver disease. Nonalcoholic fatty liver disease is divided into two types: isolated fatty liver, in which only fat accumulates, and nonalcoholic steatohepatitis, in which fat, inflammation, and liver cell damage accumulate. NASH does not usually cause symptoms. Most people with NASH feel fine and are unaware that they have it. As NASH progresses and liver damage worsens, a patient may experience fatigue, weight loss, general weakness, and acne in the upper right part of the belly. Screening for nonalcoholic fatty liver disease in the general population is not advised. It is usually considered after discovering unexplained liver enzyme levels or imaging reveals hepatic steatosis. A patient’s medical history, a physical exam, and tests for nonalcoholic steatohepatitis are used by doctors.
Want to discover how the Nash treatment pipeline will look like? Request for sample @ NASH Drug Pipeline 2023
A snapshot of the Nonalcoholic Steatohepatitis Pipeline Drugs mentioned in the report:
|
Drugs |
Company |
Phase |
MoA |
RoA |
|
Resmetirom |
Madrigal Pharmaceuticals |
Phase III |
Thyroid hormone receptor beta agonists |
Oral |
|
Aramchol |
Galmed Research and Development, Ltd. |
Phase III |
stearoyl-CoA desaturase 1 (SCD1) modulator |
Oral |
|
Lanifibranor |
Inventiva |
Phase III |
Peroxisome proliferator-activated receptor alpha agonist |
Oral |
|
BI 456906 |
Boehringer Ingelheim |
Phase II |
Glucagon like peptide 1 receptor agonist |
Subcutaneous |
|
BIO89-100 |
89bio, Inc. |
Phase II |
FGF21 Stimulants |
Subcutaneous |
|
ORMD-0801 |
Oramed Pharmaceuticals |
Phase II |
Ornithine decarboxylase stimulant |
Oral |
|
EYP001 |
ENYO Pharma |
Phase II |
Farnesoid X-activated receptor agonist |
Oral |
|
TERN-501|TERN-101 |
Terns Pharmaceuticals |
Phase II |
Thyroid hormone receptor beta agonist |
Oral |
|
Belapectin |
Galectin Therapeutics Inc. |
Phase II/III |
Galectin 3 inhibitors |
Intravenous |
|
Cotadutide solution |
AstraZeneca |
Phase II/III |
GLP-1/glucagon dual agonist |
Subcutaneous |
|
AZD 2693 |
AstraZeneca |
Phase I |
Adiponutrin inhibitor |
Subcutaneous |
|
AMG 609 |
Amgen |
Phase I |
RNA interference |
Subcutaneous |
|
LY-3849891 |
Eli Lilly and Company |
Phase I |
RNA interference |
Subcutaneous |
|
TJC0265 |
TaiwanJ Pharmaceuticals Co., Ltd |
Preclinical |
Autotaxin inhibitors |
NA |
|
LR19131 |
LG Chem |
Preclinical |
NA |
NA |
|
GMA-107 |
Gmax Biopharm |
Preclinical |
NA |
Parenteral |
|
VK-1430 |
Viking Therapeutics |
Preclinical |
Diacylglycerol O acyltransferase inhibitor |
NA |
|
RXC007 |
Redx Pharma |
Preclinical |
Rho-associated kinase inhibitors |
Oral |
Want to know how NASH dash the pipeline race is for new agents? Download the sample page @ NASH Clinical Trials
Nonalcoholic Steatohepatitis Therapeutics Assessment
The nonalcoholic steatohepatitis pipeline report proffers an integral view of the nonalcoholic steatohepatitis emerging novel therapies segmented by stage, product type, molecule type, mechanism of action, and route of administration.
Scope of the Nonalcoholic Steatohepatitis Pipeline Report
- Coverage: Global
- Nonalcoholic Steatohepatitis Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Nonalcoholic Steatohepatitis Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- NASH Therapeutics Assessment By Route of Administration: Intra-articular, Intraocular, Intrathecal, Intravenous, Ophthalmic, Oral, Parenteral, Subcutaneous, Topical, Transdermal
- NASH Drugs Assessment By Molecule Type: Oligonucleotide, Peptide, Small molecule
- NASH Therapies Assessment By Mechanism of Action: Adiponutrin inhibitor, Diacylglycerol O acyltransferase inhibitor, Adiponutrin modulator, RNA interference, Glucagon like peptide 1 receptor agonist, Ornithine decarboxylase stimulant, Farnesoid X-activated receptor agonist, Thyroid hormone receptor beta agonist, Mitochondrial membrane transport protein modulator, Thyroid hormone receptor beta agonists, Peroxisome proliferator-activated receptor alpha agonist, Autotaxin inhibitors, Rho-associated kinase inhibitors, FGF21 Stimulants, GLP-1/glucagon dual agonist
- Key Nonalcoholic Steatohepatitis Companies: Madrigal Pharmaceuticals, Intercept Pharmaceuticals, Cirius Therapeutics, Novo Nordisk, Inventiva, Galmed Pharmaceuticals, AstraZeneca, Galectin Therapeutics, Viking Therapeutics, Eli Lilly and Company, Terns Pharmaceuticals, Sinew Pharma, 89bio, Inc., Enanta Pharmaceuticals, Inc, Eccogene, Novartis Pharmaceuticals, Poxel SA, AngioLab, Pfizer, Arrowhead Pharma, LG Chem, Redx Pharma, Lipocine, Inc., CytoDyn, Inc., Alnylam Pharmaceuticals, Inc., Chemomab Therapeutics, NuSirt Biopharma, HK inno. N, Aligos Therapeutics, Altimmune, Inc., Kowa Pharmaceutical, Ionis Pharmaceuticals, NorthSea Therapeutics, Rivus Pharmaceuticals, Hanmi Pharmaceutical, Hepagene Therapeutics, HighTide Biopharma, Akero Therapeutics, Merck Sharp & Dohme LLC, Cascade Pharmaceuticals, Hepion Pharmaceuticals, Chipscreen Biosciences, Boston Pharmaceuticals, Bristol-Myers Squibb, Sunshine Lake Pharma, GSK plc., Future Medicine, Gilead Sciences, ENYO Pharma, Histogen, and others
- Key Nonalcoholic Steatohepatitis Pipeline Therapies: ERN-101, SNP-610, LJN452 (tropifexor + licogliflozin), PXL065, ALS-L1023, PF-06865571 + PF-05221304, PF-06835919, PF-06865571, ORMD-0801, Norucholic acid, NNC0194-0499, MN-001, MK-3655, MET642, MET409, LPCN 1144, LIK066, Leronlimab, ALN-HSD, RXC007, LR19018, LR19131, CM101, Leu-Mit-Sil (NS-0200), IN-A010, Efruxifermin (EFX), Efinopegdutide, JKB-122, CS0159 (Linafexor), CRV431, Chiglitazar sodium, ARO-PNPLA3, ECC4703, BOS-580, HEC96719, GSK4532990, BIO89-100, Cotadutide solution, Firsocostat, EPY001a, Emricasan, BMS-986036, BIO89-100, BI 456906, BFKB8488A, AXA1125, ASC 41, GS-9674 (Cilofexor), ZSP1601, and others
Dive deep into rich insights for nonalcoholic steatohepatitis research; visit @ NASH Treatment Pipeline
Table of Contents
|
1. |
Nonalcoholic Steatohepatitis Pipeline Report Introduction |
|
2. |
Nonalcoholic Steatohepatitis Pipeline Report Executive Summary |
|
3. |
Nonalcoholic Steatohepatitis Pipeline: Overview |
|
4. |
Analytical Perspective In-depth Commercial Assessment |
|
5. |
Nonalcoholic Steatohepatitis Clinical Trial Therapeutics |
|
6. |
Nonalcoholic Steatohepatitis Pipeline: Late Stage Products (Pre-registration) |
|
7. |
Nonalcoholic Steatohepatitis Pipeline: Late Stage Products (Phase III) |
|
8. |
Nonalcoholic Steatohepatitis Pipeline: Mid Stage Products (Phase II) |
|
9. |
Nonalcoholic Steatohepatitis Pipeline: Early Stage Products (Phase I) |
|
10. |
Nonalcoholic Steatohepatitis Pipeline Therapeutics Assessment |
|
11. |
Inactive Products in the Nonalcoholic Steatohepatitis Pipeline |
|
12. |
Company-University Collaborations (Licensing/Partnering) Analysis |
|
13. |
Key Companies |
|
14. |
Key Products in the Nonalcoholic Steatohepatitis Pipeline |
|
15. |
Unmet Needs |
|
16. |
Market Drivers and Barriers |
|
17. |
Future Perspectives and Conclusion |
|
18. |
Analyst Views |
|
19. |
Appendix |
About DelveInsight
DelveInsight is a leading Business Consultant, and Market Research firm focused exclusively on life sciences. It supports Pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Ankit Nigam
Email: Send Email
Phone: +19193216187
Address:304 S. Jones Blvd #2432
City: Albany
State: New York
Country: United States
Website: https://www.delveinsight.com/consulting